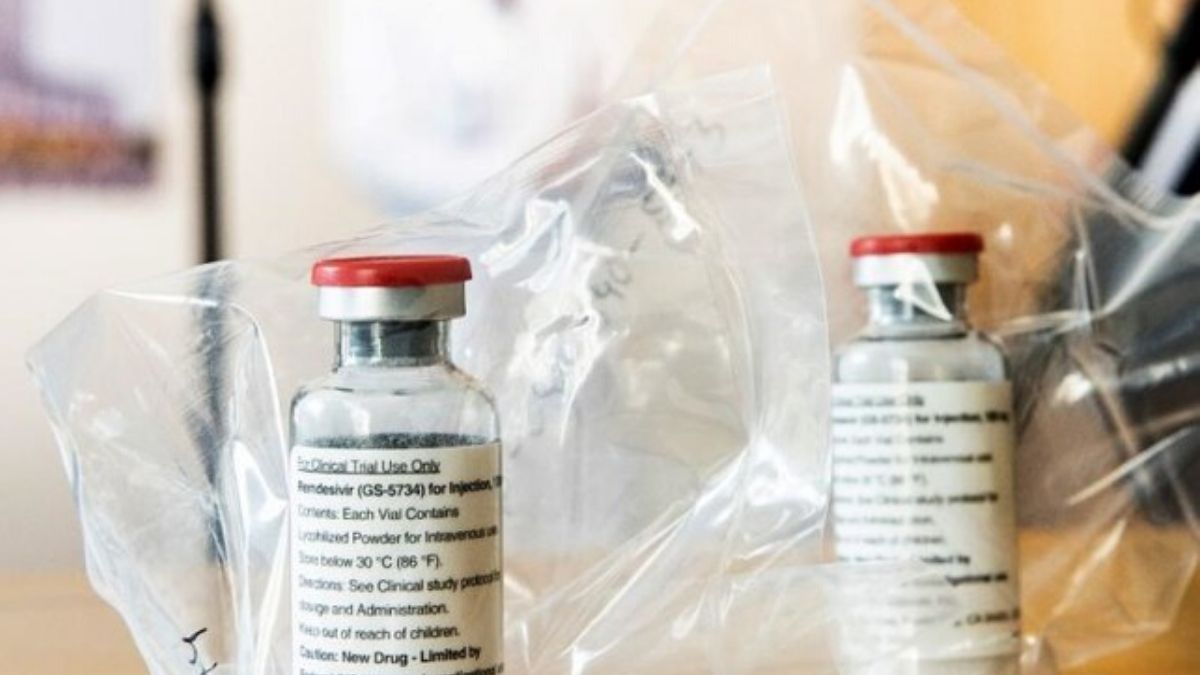
The Drug Regulatory Authority of Pakistan (Drap) said on Monday that remdesivir, a medication which has demonstrated viable against coronavirus (Anti-covid Drug), will be accessible in the nation inside three weeks.
(CEO) Asim Rauf revealed to Dawn that Drap, after enrollment of the medication, had permitted two pharmaceutical organizations to import this basic medication. The controller has offered licenses to 14 makers for delivering the medication at home.
Remdesivir infusion is an antiviral which prevents the infection from imitating itself or hinders its capacity to do as such.
The medication has been affirmed for use in the United States, Japan, South Korea and the European Union after its clinical preliminaries ended up being empowering.
Must Read : China’s Covid 19 vaccine approved for military use
This is a similar medication which government Railways Minister Sheik Rashid Ahmed had referenced at a question and answer session after he recuperated from Covid-19.
Portraying coronavirus as an unpleasant malady, Sheik Rashid had lamented that the infusion vital for treatment of basic patients was not accessible in any event, for Rs500,000 to a clergyman who had held 14 services in his political profession.
“I thank the executive of the National Disaster Management Authority for organizing this infusion,” the clergyman had expressed. “I ask that nobody, not even my adversary, experiences this ailment.”
The Drap executive surrendered that the medication was not effectively accessible seven days prior, however the circumstance had now improved and following three weeks adequate stocks would land in the market.
Be that as it may, he explained, remdesivir would not be sold over the counter at drug stores. “Rather, it will be given to basic patients by medical clinics concerned or legitimately by chose pharmaceutical organizations.” He said Pakistan was the 6th nation on the planet which had jumped upon the chance to make this medication available to patients.
Remdesivir was affirmed by the US Food and Drug Administration (FDA) on May 1. A deficiency at that point stood up to the FDA, however with the progression of time the circumstance improved.
At a meeting on June 25, a five-judge Supreme Court seat, headed by Chief Justice Gulzar Ahmed, was educated that the National Disaster Management Authority (NDMA) had looked for endorsement from Drap for the import of 7,000 vials of remdesivir infusion, which was then unregistered.
The controller, through its letter of June 9, had given a NOC to the NDMA with the condition that the last should submit subtleties of patients, including their CNIC numbers and solutions.
The controller had additionally expressed that it would not be liable for the quality, security and adequacy of the medication as the medication was not enrolled with the power.
The endorsement was given under Rule 13 of Drugs Import and Export Rules of 1976, which required earlier endorsement for the import of a medication.
The Supreme Court requested Drap to quick track enlistment of the medications which had just been enrolled in the West and were being utilized for the treatment of Covid-19. The court said the import of those medications ought to be encouraged which were not made in the nation and later neighborhood creation under permit be energized. The pinnacle court had additionally observed corrupt components out to store the counter infection synthetic concoctions, life-sparing medications and gear used to distinguish, screen and treat patients influenced by Covid-19. The court had lamented that fundamental instruments like dosimeters and oxygen chambers had vanished from the market and were being sold at excessive costs.
The court had requested the administrative specialists, law implementation offices and wellbeing services of the bureaucratic just as common governments to make a move against hoarders of these basic wares.